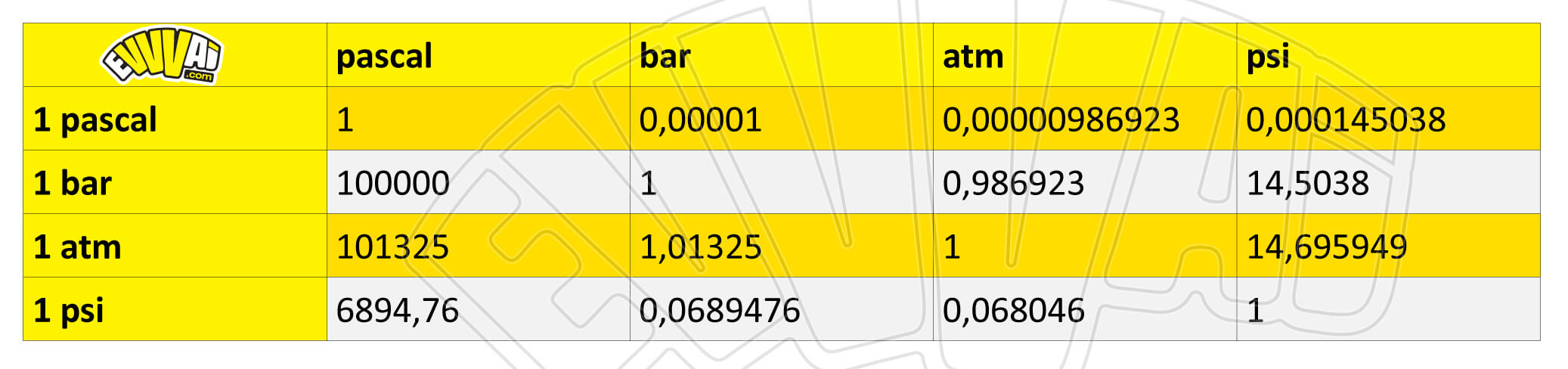
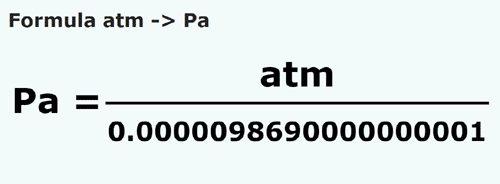
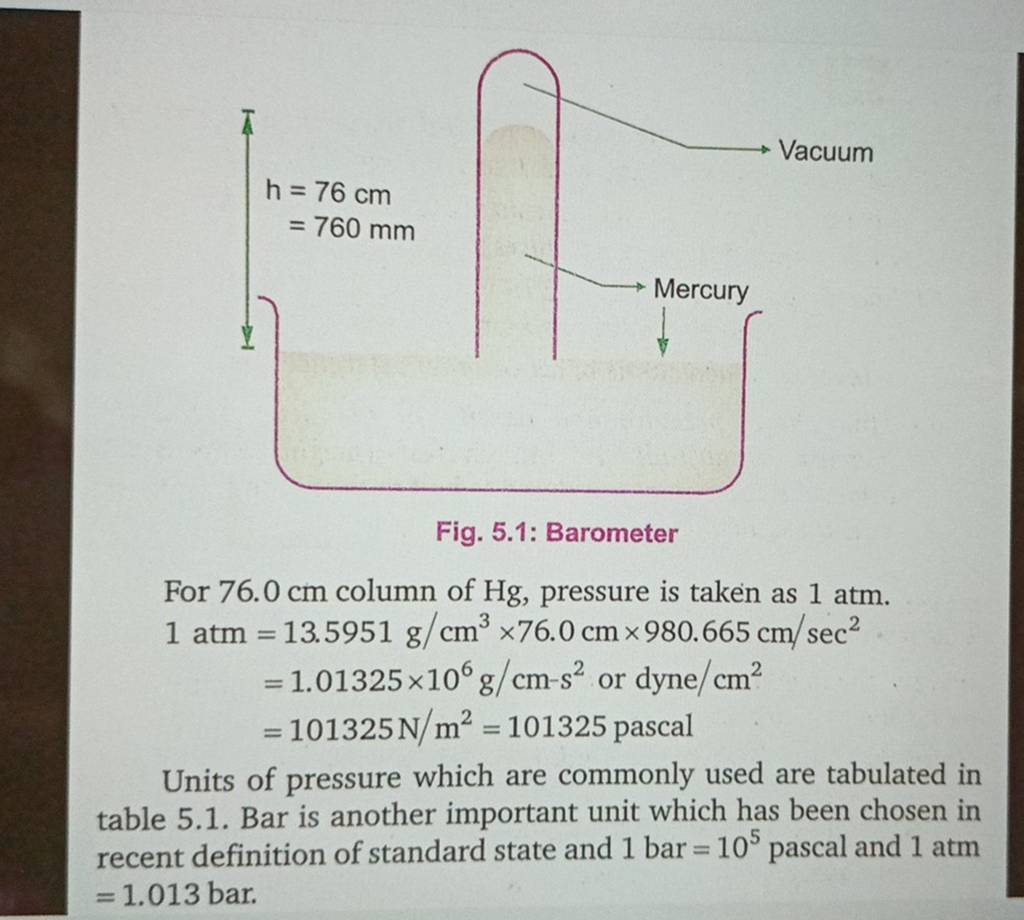
SOLVED: 1 atm = 760 mm Hg 760 torr = 1 atm 1 atm = 101325 Pa 101.3 J = 1 L atm 1 nm = 1 x 10^-9 m 0.08212 M^-1

Atmospheres and Conversions: 1.00 atm = x 10 5 Pa = kPa = 760. Torr = 14.7 psi To convert pressure: 1.Turn what you have to atm 2.Multiply. - ppt download
3. For a solution if pA 600 mm Hg. PB 840 mm Hg under atmospheric conditions and vapourpressure of solution is 1 atm then find(i) Composition of solution(i) Composition of vapour in

the SI unit of pressure is the pascal, pa, but in the V-P relationship of gases, it is necessary to be - Brainly.ph

Gas Laws Chapter 5. Pressure Force per unit area Measured in Atmospheres ( atm) Mm of Hg = Torr Pascals or kiloPascals (Pa or kPa) - ppt download

![Tamil] In the SI system 1 atm= pascal. Tamil] In the SI system 1 atm= pascal.](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/8414727.webp)